Learning Outcomes
By the end of this lesson, students will be able to:
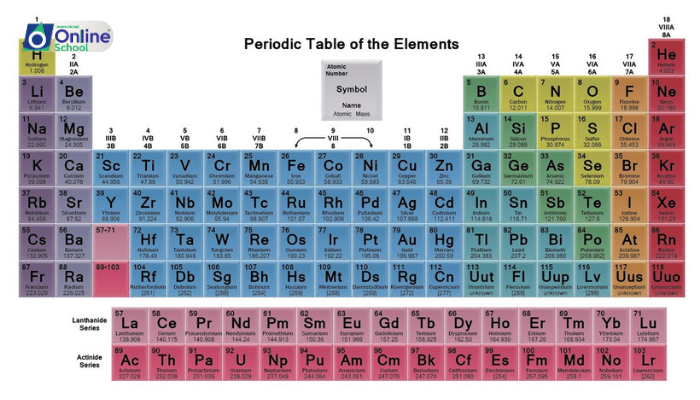
i. Define and identify different element families, also known as groups, within the periodic table.
ii. Determine the location of specific element families, such as alkali metals, alkaline earth metals, halogens, and noble gases, within the periodic table.
iii. Recognize and explain the common characteristics and trends observed among elements within each family.
iv. Analyze how the position of an element within a family relates to its chemical properties and reactivity.
v. Apply the knowledge of element families to predict and explain the behavior of elements in chemical reactions.
Introduction
The periodic table, a treasure trove of information about elements, is not merely a collection of isolated entities. It harbors distinct groups of elements, known as element families, that share remarkable similarities in their properties and behavior.
i. Element Families: A Tale of Shared Electron Configurations
Element families, also known as groups, are vertical columns in the periodic table that encompass elements with similar valence electron configurations, the outermost electrons involved in chemical bonding. This shared electron arrangement leads to a striking uniformity in their chemical properties.
ii. Alkali Metals: A Glimmer of Reactivity
Alkali metals, occupying Group 1 of the periodic table, are highly reactive metals characterized by the presence of a single valence electron in their outermost s orbital. Their low ionization energies and large atomic radii make them highly electropositive, readily losing their valence electron to form ionic compounds.
iii. Alkaline Earth Metals: A Step Further
Alkaline earth metals, residing in Group 2 of the periodic table, share similarities with alkali metals but possess two valence electrons in their outermost s orbital. Their reactivity is slightly lower than that of alkali metals, reflecting their higher ionization energies.
iv. Halogens: Masters of Electron Acquisition
Halogens, inhabiting Group 17 of the periodic table, are nonmetallic elements with seven valence electrons, one less than a full outer shell. Their strong electronegativity drives their tendency to gain electrons, forming ionic compounds with metals and covalent bonds with nonmetals.
v. Noble Gases: A Realm of Stability
Noble gases, nestled in Group 18 of the periodic table, are the most chemically inert elements. With their outer electron shells completely filled, they have no need to gain or lose electrons, making them exceptionally stable and unreactive.
vi. Group Characteristics: A Unifying Theme
Elements within each family exhibit common trends in their properties:
Ionization Energy: Ionization energy, the energy required to remove an electron, generally decreases down a group.
Electronegativity: Electronegativity, the measure of an atom's ability to attract electrons, generally increases across a period from left to right and decreases down a group.
Atomic Radii: Atomic radii, the measure of an atom's size, generally increase down a group.
vii. Position Within a Family: A Predictor of Behavior
The position of an element within a family provides valuable insights into its chemical behavior:
Reactivity: Elements within a family exhibit similar reactivities due to their shared electron configurations.
Bonding Patterns: The type of bonds formed by elements within a family is often predictable based on their position in the periodic table.
Chemical Reactions: The behavior of elements in chemical reactions can be predicted to some extent based on their family membership.
Element families, the distinctive vertical groups within the periodic table, offer a deeper understanding of the similarities and trends in element properties. By delving into the concept of shared electron configurations and group characteristics, we gain valuable insights into the chemical behavior of elements, enabling us to predict and explain their interactions and reactions, further illuminating the intricate tapestry of chemistry.